These combustion products are formed when air and fuel are mixed in the optimum proportion (14,7:1). But, unfortunately, this ratio is not always maintained, and therefore there are harmful substances in the exhaust gases.
The Fiesta is equipped with a controlled three-way catalytic converter, the diesel engine is equipped with an oxidizing catalytic converter
Without exception, all vehicles are equipped with a controlled three-way catalytic converter, vehicles with Endura-DE diesel engines are equipped with an oxidizing catalytic converter. A controlled catalytic converter reduces carbon oxides by approximately 85%, hydrocarbons by 80%, nitrogen oxides by 70%. Oxidation catalytic converters have no effect on the concentration of nitrogen oxides. With increasing mileage, the efficiency of the catalytic converter decreases. Designation «controlled» indicates that when the engine is running, the composition of the exhaust gases is constantly monitored using an oxygen concentration sensor and the content of harmful substances in the gases is reduced to the standards prescribed by law.
Oxygen sensor function (Lambda probe)
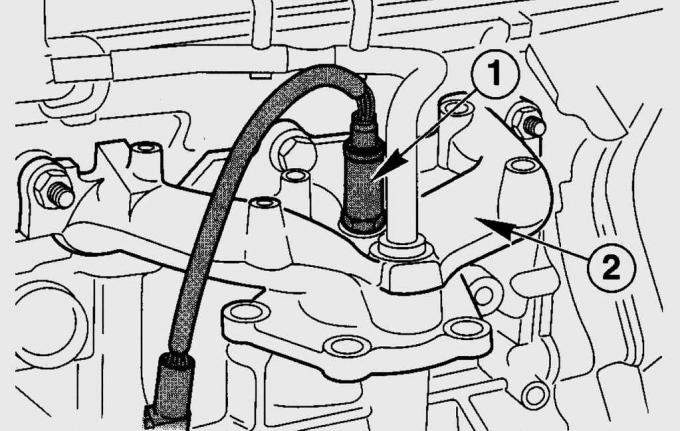
Pic. 11.4. Sensor location (1) oxygen concentration in the exhaust pipe (2), where the temperature of the exhaust gases is maximum
Oxygen concentration sensor (HO2S) on a Fiesta, installed in front of the catalytic converter in the front exhaust pipe (pic. 11.4) and operates on the principle of a galvanic cell with a solid electrolyte in the form of a ceramic material made of zirconium dioxide and yttrium oxide. The ceramic material of the sensor is exposed to the exhaust gases from the outside, its inner surface is connected to the ambient air. To reduce the time it takes to bring the sensor into normal operating mode, it is equipped with electrical heating. Due to the difference in the oxygen content in the exhaust gases and the ambient air, a potential difference arises in the sensor, which greatly increases at a certain residual oxygen content in the exhaust gases. This voltage jump occurs exactly at the ratio of fuel and air l=1. With a lack of oxygen (l<1), i.e. with a rich air-fuel mixture, the voltage is 0.9–1.1 V. With a lean mixture (l>1) the voltage drops to 0.1 V.
The signal from the oxygen concentration sensor is transmitted to the fuel injection system control unit. The unit enriches or leans the air-fuel mixture to keep the fuel-to-air ratio as close as possible to the optimum l=1.
Working area of the catalytic converter
The efficiency of a catalytic converter is a function of the operating temperature. The converter begins to work at a temperature of approximately 300°C, which is reached after 25–30 seconds of movement. The operating temperature in the range of 400–800°C provides optimal conditions for obtaining maximum efficiency and a long service life of the converter.
The ceramic catalytic converter is susceptible to extreme heat. If its temperature exceeds 900°C, the process of intensive aging begins, and at temperatures above 1200°C, its performance is completely impaired.
The active layer consists of metals that are sensitive to the lead content in the fuel, the deposition of which rapidly reduces the activity of the catalytic layer. Therefore, engines with catalytic converters should only be run on unleaded petrol.
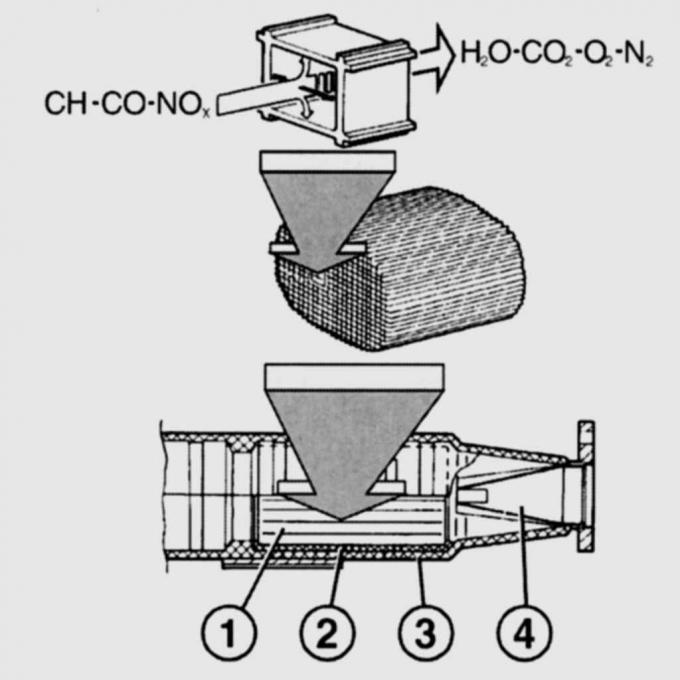
Pic. 11.5. Diagram of the operation of a catalytic converter. NOx emissions from the engine (nitrogen oxides), CO (carbon monoxide) and CH (hydrocarbons), and after the reaction in the catalytic converter, N2 (nitrogen), CO2 (carbon dioxide) and H2O (water): 1,2 - metal grids; 3 - body; 4 - perforated funnel
The catalytic converter has a porous ceramic base coated with precious metals - platinum and rhodium and enclosed in a stainless steel shell. The ceramic base, located on a wire mesh, is penetrated by a large number of parallel channels. An intermediate layer is applied on the walls of the channels to increase the active surface of the catalytic converter (pic. 11.5).
The catalytic converter contains 2-3 g of precious metals, with platinum contributing to the oxidation, and rhodium to the reduction of nitrogen oxides.
The catalytic converter neutralizes harmful substances such as carbon monoxide, hydrocarbons and nitrogen oxides (that's why it's called a three-way catalytic converter).
PRACTICAL ADVICE
Operation of vehicles with a catalytic converter
• If the Fiesta does not start due to a dead battery, do not attempt to start the engine by pushing or towing the vehicle. A lot of unburned fuel will get into the catalytic converter, which will eventually make it unusable.
• In the event of misfiring or misfiring, check the ignition system immediately and avoid high engine speeds when driving further.
• Close the catalytic converter carefully before applying protective mastic to the underbody, otherwise fire may occur.
• Be sure to check the heat shields every time the vehicle is lifted.
• Leakage of system of release of the fulfilled gases (burnt gasket, crack from high temperature, etc.) in front of the oxygen concentration sensor leads to incorrect measurement results (high proportion of oxygen). Therefore, the ECM will enrich the mixture, which will lead to increased fuel consumption and premature wear of the catalytic converter.
TECHNICAL DICTIONARY
Composition of exhaust gases
carbon monoxide (carbon monoxide - CO).
The richer the air-fuel mixture, the more carbon monoxide is produced. Precise control of the amount of injected fuel, correctly set ignition timing and even distribution of the mixture in the combustion chamber reduce the content of carbon monoxide in the exhaust gases. Never measure carbon monoxide indoors, as carbon monoxide is poisonous and even small concentrations indoors can be fatal. In air, carbon monoxide combines relatively quickly with oxygen to form carbon dioxide. Although carbon dioxide is not poisonous, it contributes to the formation «greenhouse» effect.
hydrocarbons (CH).
Hydrocarbon compounds are grouped together. CH content depends on engine design (immutable value). Too rich or too lean air-fuel mixture also increases the proportion of CH content in the exhaust gases. Some of them are safe, others can cause cancer. All hydrocarbon compounds together with nitrogen oxides (NOx) form smog (poorly soluble fog clouds of exhaust gases).
nitrogen oxides (NOx or NO) -
are formed primarily due to the presence of nitrogen in the air entering the combustion chamber (over 3/4). Their concentration is especially high in engine designs with low fuel consumption and low CO and CH content in the exhaust gases. These engines are characterized by high combustion temperatures and a lean air-fuel mixture. At high concentrations, nitrogen oxides can damage the respiratory system. When combined with water, acid rain is formed.
Carbon dioxide (CO2).
It is formed during the combustion of fuel containing carbon, when combined with atmospheric oxygen. Carbon dioxide reduces the beneficial effect of the Earth's ozone layer, which protects against harmful ultraviolet radiation from the sun.
Toxic substances contained in the exhaust gases of diesel engines.
During the operation of a diesel engine, a small amount of CO and CH is formed. Due to the higher compression, the diesel engine emits less nitrogen oxides. But the diesel engine is characterized by other harmful substances in the combustion products. For example, soot is a typical component of diesel exhaust gases. Soot is made up of unburned carbons and ash. Soot particles, when inhaled into the respiratory organs, become the causative agents of cancer. Sulfur dioxide (SO2) also formed in the presence of sulfur, primarily in diesel fuel. Contributes to the appearance of sulfuric or sulphurous acid in the rain (acid rain). Diesel vehicles cause 3% of acid precipitation.
Carbon dioxide is formed during the combustion of diesel fuel only at higher concentrations.
Visitor comments